What Is The Electron Configuration Of The Calcium Ion
Camila Farah
So that would be 18 electrons.
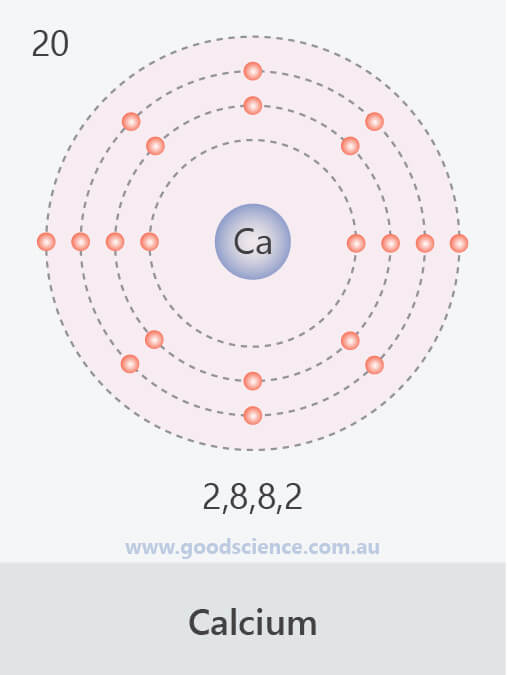
The electron configuration for calcium cl is 1s2 2s2 2p6 3s2 3p5. In this video we will write the electron configuration for ca2 the calcium ion. Thus the electron configuration of a ca 2 ion is. 1s 2 2s 2 2p 6 3s 2 3p 6 which is isoelectronic with the noble gas argon.
It loses two electrons when it forms an ion. Since 1s can only hold two electrons the next 2 electrons for calcium go in the 2s orbital. Neutral calcium s electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. The calcium ion ca 2 would in reality be ar argon.
The electron configuration of a calcium atom would be but the calcium ion has two electrons less than a calcium atom. So just write the electron configuration for ar. A neutral calcium atom has 20 electrons as indicated by its atomic number. The p orbital can hold up to six electrons.
RELATED ARTICLE :
- what to do when female cat is in heat
- what to do when you forgot your iphone passcode
- what to do when a guy kisses your neck
Ca2 is the ion of calcium which means that it has 2 less electrons than neutral calcium. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. In writing the electron configuration for calcium the first two electrons will go in the 1s orbital. 1s2 2s2 2p6 3s2 3p6.
So it would be 1s2 2s2 2p6 3s2 3p6. Calcium has 20 electrons but is in group two so wants to lose 2 electrons. We ll also look at why calcium forms a 2 ion and how the electron configur.
Source : pinterest.com













